The Accidental Discovery of Teflon
A polymer is a material with a high molecular weight that is formed from simple molecules called monomers. Polymers may be either natural or synthetic in origin. we know synthetic polymers, such as polyethylene and nylon. Another synthetic polymer is Teflon, which was discovered quite by accident.
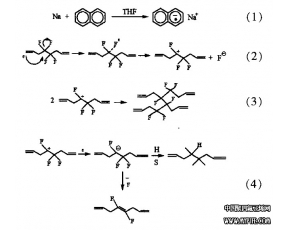
In 1938 a scientist at Du Font named Roy J. Plunkett made a rather curious observation: A tank of the gaseous compound tetrafluoroethylene, CF2=CF2, that was supposed to be full seemed to have no gas in it. Rather than discarding the tank, Plunkett decided to explore further by cutting the tank open. He found that the inside of the tank was coated with a waxy white substance that was remarkably unreactive toward even the most corrosive chemical reagents. The compound was formed by the addition polymerization of tetrafluoroethylene:
nCF2==CF2─poIymerizLition→─(CF2 -CF2)n─
As it turned out, the properties of Teflon were ideal for an immediate and important application in the development of the first atomic bomb. Uranium hexafluoride, UF6, which was used to separate fissionable 235U by gaseous diffusion , is an extremely corrosive material. Teflon was used as a gasket material in the gaseous diffusion plant. It is now used in a variety of applications, from nonstick cookware to space suits.
Plunkett's desire to know more about something that just didn't seem right is a wonderful example of how natural scientific curiosity can lead to remarkable discoveries. If you wish to read about more such accidental discoveries, we recommend Royston M. Roberts,Serendipity: Accidental Discoveries in Science, John Wiley and Sons, 1989.